Given :
A copper rod has a length of 1.7 m and a cross-sectional area of
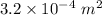 .
.
One end of the rod is in contact with boiling water and the other with a mixture of ice and water.
To Find :
The mass of ice per second that melts.
Solution :
Amount of heat transfer from boiling water to ice through the copper bar is :
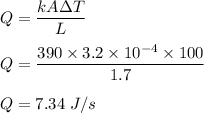
Let, amount of ice melts per second is :
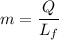
Here,
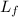 is the latent of fusion of water and is equal to
is the latent of fusion of water and is equal to
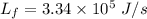 .
.
Putting all values in above equation, we get :
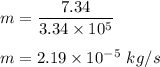
Hence, this is the required solution.