Answer: 116 g of copper
Step-by-step explanation:
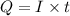
where Q= quantity of electricity in coloumbs
I = current in amperes = 24.5A
t= time in seconds = 4.00 hr =
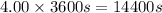 (1hr=3600s)
(1hr=3600s)
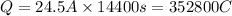
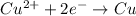
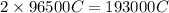 of electricity deposits 63.5 g of copper.
of electricity deposits 63.5 g of copper.
352800 C of electricity deposits =
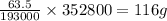 of copper.
of copper.
Thus 116 g of Cu(s) is electroplated by running 24.5A of current
Thus remaining in solution = (0.1-0.003)=0.097moles