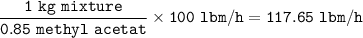The mixture flow rate in lbm/h = 117.65 lbm/h
Further explanation
Given
15.0 wt% methanol
The flow rate of the methyl acetate :100 lbm/h
Required
the mixture flow rate in lbm/h
Solution
mass of methanol(CH₃OH, Mw= 32 kg/kmol) in mixture :

mass of the methyl acetate(C₃H₆O₂,MW=74 kg/kmol,85% wt) in 200 kg :
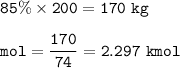
Flow rate of the methyl acetate in the mixture is to be 100 lbm/h.
1 kg mixture = 0.85 .methyl acetate
So flow rate for mixture :