Solution :
It is given that dilute hydrochloric acid is titrated with sodium carbonate solution.
 of a 0.100
of a 0.100
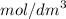 of hydrocholric acid is used.
of hydrocholric acid is used.
Thus, 10 mL of the 0.1 molar dilute hydrochloric acid is titrated with 16.2 mL of Sodium carbonate solution.
The equation would be :

Now we know that that the
Molarity =
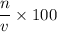
For hydrochloric acid solution,
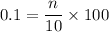
∴ n = 0.01 moles.
Now for one mole of
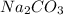 , two moles of HCl acid is used.
, two moles of HCl acid is used.
For one mole of HCl, one-half mole of
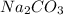 is required.
is required.
Therefore, for 0.01 mole of HCl, we require 0.005 mole of
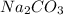 .
.
Hence, 0.01 mole of hydrochloric acid and 0.005 mole of
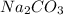 solution is used.
solution is used.
The color turns red when methyl orange indicator is used in HCl acid.