Answer:
Approximately
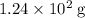 , assuming that all sulfur in that coal was converted to
, assuming that all sulfur in that coal was converted to
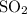 .
.
Step-by-step explanation:
Look up the relative atomic mass of
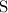 and
and
 on a modern periodic table:
on a modern periodic table:
Convert the unit of the mass of coal to grams:
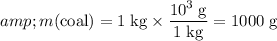 .
.
Mass of sulfur in that much coal:
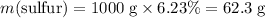 .
.
The relative atomic mass of sulfur is
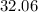 . Therefore, the mass of each mole of sulfur atoms would be
. Therefore, the mass of each mole of sulfur atoms would be
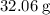 . Calculate the number of moles of atoms in that
. Calculate the number of moles of atoms in that
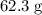 of sulfur:
of sulfur:
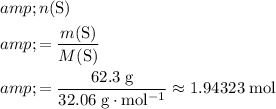 .
.
Each
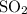 molecule contains one sulfur atom. Therefore, assuming that all those (approximately)
molecule contains one sulfur atom. Therefore, assuming that all those (approximately)
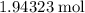 of sulfur atoms were converted to
of sulfur atoms were converted to
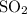 molecules through the reaction with
molecules through the reaction with
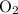 , (approximately)
, (approximately)
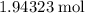 of
of
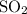 molecules would be produced.
molecules would be produced.
Calculate the mass of one mole of
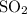 molecules:
molecules:
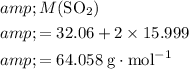 .
.
The mass of that
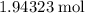 of
of
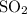 molecules would be:
molecules would be:
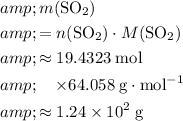 .
.