Mass of NaCl L 13.75 g
Further explanation
Given
The solution of 5.5% by mass
mass of commercial bleach solution : 250 g
Required
mass of NaCl
Solution
Percent mass (% w/w) = percent mass of solute in 100 g mass of solution
Solution = solute + solvent
Solute = NaCl
Solution = Commercial bleach
Can be formulated :
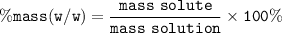
Input given values in formula :
