Answer:
D) Mg .
Step-by-step explanation:
Hello!
In this case, since compounds with the formula X3N2 suggest that the oxidation state of X is +2 because the oxidation state of nitrogen is -3 as it gains three electrons in order to attain a complete octet; among the given options, just magnesium (option D) Mg ) has such charge as it is in group 2A, which suggests it loses two electrons when forming ionic compounds:
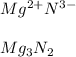
Best regards!