Answer:
T₂ = 421.4 K
Explanation:
The temperature, T, of a given mass of gas varies inversely with its Volume,V.
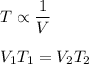
We have,
V₁ = 98 cm³, T₁ = 28° C = (28+273) K = 301 K
V₂ = 70 cm₃
We need to find the new temperature of the gas. Using the above relation to find T₂.
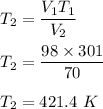
Hence, the required temperature of the gas is 421.4 K.