Answer:
True.
Step-by-step explanation:
Hello!
In this case, since the law of conservation of mass and energy states that both mass and energy cannot be neither created nor destroyed, if we want to analyze it in a chemical reaction, it is necessary to realize that the initial reactants are composed by a specific amount of atoms of each element and therefore the products must have that equal amount. For instance, going over the formation of water from hydrogen and oxygen:
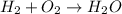
We can see hydrogen are equal at both sides whereas oxygen do not; that is why we balance it by putting a coefficient of 2 just before hydrogen and water in order to equal the new 4 atoms of H and 2 atoms of O at each side.
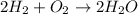
That is why this statement is true.