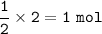Moles of O₂ are required : 1 mol
Further explanation
Given
2.00 moles of H₂
Required
moles of O₂
Analysis
Balanced equation
Find mol ratio O₂ and H₂
Solution
Balanced equation
2H₂ + O₂ → 2H₂O
From the equation, the mol ratio of H₂ : O₂ = 2 : 1, so mol O₂ :