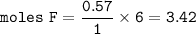There are 3.42 moles of F in 0.570 moles of C₃H₂F₆
Further explanation
Given
0.570 moles of C₃H₂F₆
Required
moles of F
Solution
The chemical formula of a compound shows the mole ratio of the constituent elements
In the compound C₃H₂F₆, the mole ratio of the elements is:
C: H: F = 3: 2: 6
So in 1 mol of C₃H₂F₆, there are 3 moles of C, 2 moles of H and 6 moles of F
And for 0.570 mol of C₃H₂F₆ :