Answer:

Step-by-step explanation:
The mass number is found by adding up the nucleons in an atom.
The nucleons are the subatomic particles found in the nucleus, so just protons and neutrons.
There are 11 protons and 12 neutrons.
Add them together.
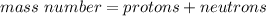
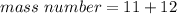
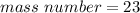
The mass number for this atom is 23.