Rate of consumption of N₂ = - 1.25 x 10⁻⁴
Further explanation
The reaction rate (v) shows the change in the concentration per unit time.

The rate reaction for Haber Bosch :
N₂(g)+3H₂(g)⇒2NH₃(g)
From equation, mol ratio N₂ : NH₃ = 1 : 2, so
the rate of formation of ammonia = 2 x the rate of consumption of nitrogen.
Given
the rate of formation of ammonia gas : 2.5x10⁻⁴ M/s
the rate of consumption of nitrogen :
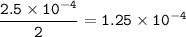
The sign negative for consumption : -1.25 x 10⁻⁴ M/s
or we can use another way:
Rate of formation of NH₃ = reaction rate x coefficient of NH₃
2.5 x 10⁻⁴ = reaction rate x 2
reaction rate = 1.25 x 10⁻⁴
Rate of consumption of N₂ = reaction rate x coefficient of N₂
Rate of consumption of N₂ = 1.25 x 10⁻⁴ x 1
Rate of consumption of N₂ = 1.25 x 10⁻⁴