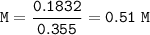The molarity of sugar in this beverage : 0.51 M
Step-by-step explanation:
Given
mass of soda=solute=33 g
volume of bottle=solution=355 ml
Required
the molarity of sugar
Analysis
- Convert mass to mol of sugar
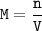
Solution
- mol of sugar-C₆H₁₂O₆(MW=180.16 g/mol g/mol)
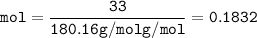
- molarity(volume=355 ml=0.355 L)