Answer:
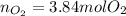
Step-by-step explanation:
Hello!
In this case, since the combustion reaction of methanol is:
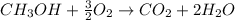
In such a way, since there is 1:3/2 mole ratio between methanol and oxygen, we can compute the moles of oxygen that are needed to burn 2.56 moles of methanol as shown below:
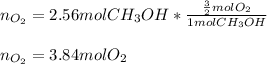
Best regards!