Answer:
Negatively charged.
Step-by-step explanation:
Hello!
In this case, since nonmetals arranged in groups 5A, 6A and 7A are able to attract the ionized form of metals (positive), we infer they are become negatively charged as they tend to gain electrons.
Some examples may be:
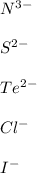
Regards!