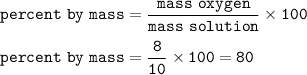The percent by mass of Oxygen : 80%
Further explanation
According to Proust: the mass percentage of the constituent elements of a compound is constant⇒compounds have a constant composition of elements
Given
mass of Oxygen (solute)= 8 grams
mass of H₂O(solution)=10 grams
Required
Percent by mass
Solution