The empirical formula for this oxide : As₂O₃
Further explanation:
Given
mass of Arsenic=3.26 g
mass of Oxygen = 1.04 g
Required
The empirical formula
Analysis
- find mole of elements
- determine the ratio of each element
Solution
mol of Arsenic(MW=74,9216 u) :
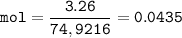
mol of Oxygen(MW=15.999 u) :
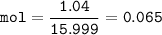 .
.
The ratio for each element (divide by smaller mol⇒Arsenic)
Arsenic : Oxygen :
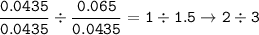
The empirical formula : As₂O₃