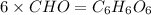Answer: The molecular formula will be
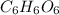
Step-by-step explanation:
Mass of
 = 17.95 g
= 17.95 g
Mass of
 = 4.87 g
= 4.87 g
Molar mass of carbon dioxide = 44 g/mol
Molar mass of water = 18 g/mol
For calculating the mass of carbon:
In 44g of carbon dioxide, 12 g of carbon is contained.
So, in 17.95 g of carbon dioxide, =
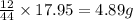 of carbon will be contained.
of carbon will be contained.
For calculating the mass of hydrogen:
In 18g of water, 2 g of hydrogen is contained.
So, in 4.87 g of water, =
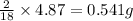 of hydrogen will be contained.
of hydrogen will be contained.
Mass of oxygen in the compound = (12.00) - (4.89+0.541) = 6.57 g
Mass of C = 4.89 g
Mass of H = 0.541 g
Mass of O = 6.57 g
Step 1 : convert given masses into moles.
Moles of C =
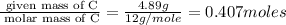
Moles of H=
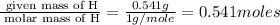
Moles of O=
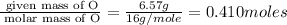
Step 2 : For the mole ratio, divide each value of moles by the smallest number of moles calculated.
For C =
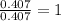
For H =
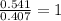
For O =
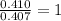
The ratio of C : H : O = 1: 1 : 1
Hence the empirical formula is
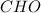 .
.
Hence the empirical formula is
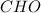
The empirical weight of
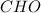 = 1(12)+1(1)+1(16)= 29 g.
= 1(12)+1(1)+1(16)= 29 g.
If 0.25 moles has mass of 44.0 g
Thus 1 mole has mass of =
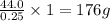
Thus molecular mass is 176 g
Now we have to calculate the molecular formula.
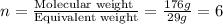
The molecular formula will be=