The given questionis incomplete. The complete question is :
The following product is not obtained during electrolysis of an aqueous solution of sodium formate
(A) H
(B)

(C) CH
(D) NaOH
Answer: (C) CH
Step-by-step explanation:
The electrolysis of solution of sodium formate is :
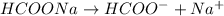
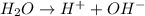
The reactions which occur at anode are:
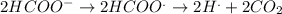
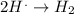
The reaction which occurs at cathode is:
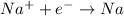
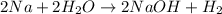
The overall reaction is:
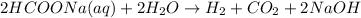
Thus CH is not obtained during electrolysis of an aqueous solution of sodium formate