Answer:

Step-by-step explanation:
First, find the molar mass of CH₄
This compound is made of carbon (C) and hydrogen (H). Look on the Peirodic Table for the masses of both elements.
- Carbon: 12.011 g/mol
- Hydrogen: 1.008 g/mol
Now, count the number of moles in the compound. There is no subscript on C, indicating 1 mole. There is a subscript of 4 on H, indicating 4 moles. We must multiply the molar mass of hydrogen by 4.
- 1 Carbon: 12.011 g/mol
- 4 Hydrogen: (4 * 1.008 g/mol)= 4.032 g/mol
Add the 2 masses.
- 12.011 g/mol + 4.032 g/mol = 16.043 g/mol
Next, find the number of moles in 35.7 grams.
Use the molar mass of CH₄ as a ratio.
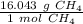
Since we want the units of grams CH₄ to cancel when we multiply, we must flip the ratio.
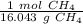
Multiply by 35.7 grams.
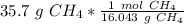
The grams of CH₄ will cancel each other out. Since there is a 1 in the numerator, we can also move 35.7 to the numerator.
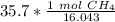
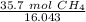
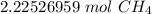
The original measurement given, 35.7 grams, has 3 significant figures (3, 5, and 7), so we must round to 3 sig figs.
For this number, it is the hundredth place.
The 5 in the thousandth place tells us to round the 2 to a 3.
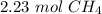
There are about 2.23 moles of CH₄ in 35.7 grams.