Answer:

Step-by-step explanation:
When converting from moles to atoms, we must use Avogadro's number. This number tells us there are 6.022 * 10²³ atoms in 1 mole. We can multiply this number by the number of moles.
First, we must set up Avogadro's number as a ratio.
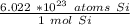
Next, multiply the number of moles by the ratio.
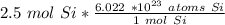
When we multiply, the moles of silicon will cancel.
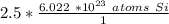
Since the denominator of the fraction is 1, we can cancel it out too.
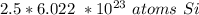
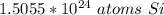
The original measurement (2.5 moles) has 2 significant figures (2 and 5). Therefore we must round to 2 sig figs. For this question, 2 sig figs is the tenth place.
The 0 in the hundredth place tells us to leave the 5 in the tenth place.
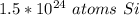
There are about 1.5 * 10²⁴ atoms of silicon.