Answer:
Limiting reactant is magnesium.
14.1 g remain unreacted.
Step-by-step explanation:
Hello!
In this case, given the balanced chemical reaction, we are able to compute the moles of each reactant by using their molar masses:
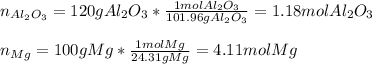
Now, since they are reacting based on a 1:3 mole ratio, we can compute the moles of magnesium consumed by the 1.18 moles of aluminum oxide as follows:
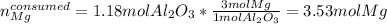
Meaning there are more available moles of magnesium than consumed, and therefore it is the limiting reactant. Moreover, it is in excess by:
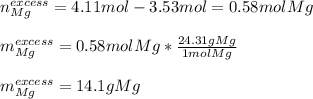
It means that 14.1 g of magnesium remain unreacted.
Regards!