Answer: A) NO increases the rate at which
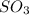 molecules are formed.
molecules are formed.
Step-by-step explanation:
A catalyst is a substance which increases the rate of a reaction by taking the reaction through a different path which involves lower activation energy and thus more reactant molecules can cross the energy barrier by undergoing collisions and convert to products.
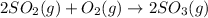
Thus NO will increase the rate of reaction by lowering the activation energy and thus the colllisions among
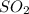 and
and
 molecules will incraese which in turn will lead to formatioon of more
molecules will incraese which in turn will lead to formatioon of more
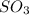 molecules.
molecules.