Answer:
Ions.
Step-by-step explanation:
Hello!
In this case, since iron is a metal which has lots of uses in the design of metallic alloys and materials, when it is at its ground state we say it is just Fe; however, since it is a metal, it is very likely to lose electrons and therefore getting positively charged as +2 or +3, say:
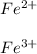
Thus, since they are positively charged, they are classified as cations, which are ions.
Best regards!