Given :
A chemical equation :
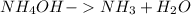
To Find :
What is the type of reaction :
A. Single replacement
B. Decomposition
C. Double replacement
Solution :
We know when a single compound turns into two or more compounds or element, this type of reaction is said to be decomposition reaction.
In the given equation
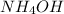 decomposes into
decomposes into
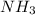 and
and
 .
.
Therefore, the given reaction is decomposition reaction.