Answer:
1.25mole of Mg(OH)₂
Step-by-step explanation:
The reaction is between Mg(OH)₂ and HCl, this is a neutralization reaction between an acid and a base.
Mg(OH)₂ + 2HCl → MgCl₂ + 2H₂O
The balanced reaction equation is given above.
2 mole of HCl reacts with 1 mole of Mg(OH)₂
So, 2.5mole of HCl will react with
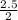 = 1.25mole of Mg(OH)₂
= 1.25mole of Mg(OH)₂
The number of moles of Mg(OH)₂ is given as 1.25mol