Answer:
Step 3 ⇒ incorrect
Further explanation:
Given
mass of Cl₂ : 4 g
Required
incorrect step
Analysis
analyze step
- determine mol of Cl₂
- mol ratio from equation
- potassium chloride formed
Solution
Reaction
2K(s)+Cl₂(g)⇒2KCl(s)
Step 1⇒correct
we determine the moles of Cl₂ by their mass
MW of Cl₂=70.9 g/mol
mol=mass : MW
Step 2 ⇒ correct
From equation, mol ratio of Cl₂ to KCl = 1 : 2, so mol KCl :
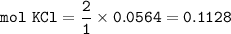
Step 3 ⇒ incorrect
Paraphrase
Step 3 ⇒ incorrect