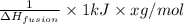Answer:
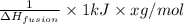
Step-by-step explanation:
Latent heat of fusion is the amount of heat required to convert 1 mole of solid to liquid at atmospheric pressure.
If
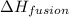 is used to melt = 1 mole of solid
is used to melt = 1 mole of solid
1 kJ of energy is used to melt =
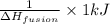
Now if molar mass of solid is x g/mol
Then mass of solid will be =