We are given the slope and the y-intercept of the line.
We can use the Slope-Intercept formula:
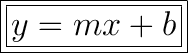
Where m is the slope, and b is the y-intercept.
Since we are given the slope and the y-intercept, we can use the formula to determine the equation of the line.
All we have to do is plug in -3 instead of m, and 5 instead of b:
y=-3x+5
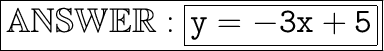
 I really hope that my answer is helpful!
I really hope that my answer is helpful!