Mass of Oxygen : 18.8 g
Further explanation
Reaction(balanced) :
4Fe + 3O₂ → 2Fe₂O₃
mass Fe = 43.7 g
mol Fe(MW= 55,845 g/mol) :
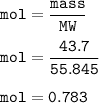
mol O₂ : mol Fe = 3 : 4, so mol O₂ :
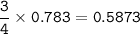
Mass O₂(MW=32 g/mol) :

Or simply you can Conservation of mass, where the masses before and after the reaction are the same
mass reactants=mass products
mass iron+mass oxygen=mass iron (III) oxide
43.7 g + mass oxygen=62.5 g
mass oxygen = 62.5 - 43.7 = 18.8 g