Answer: The half life of the sample of silver-112 is 3.303 hours.
Step-by-step explanation:
All radioactive decay processes undergoes first order reaction.
To calculate the rate constant for first order reaction, we use the integrated rate law equation for first order, which is:
![k=(2.303)/(t)\log ([A_o])/([A])](https://img.qammunity.org/2021/formulas/chemistry/college/lkf1aqszq6ll1r1vzb8j4nur3020qt55j9.png)
where,
k = rate constant = ?
t = time taken = 1.52 hrs
![[A_o]](https://img.qammunity.org/2021/formulas/physics/college/3jrctnxyrdjmiz9ngr0s6o9r3hdvpo6qhe.png) = Initial concentration of reactant = 100 g
= Initial concentration of reactant = 100 g
[A] = Concentration of reactant left after time 't' = [100 - 27.3] = 72.7 g
Putting values in above equation, we get:
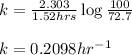
To calculate the half life period of first order reaction, we use the equation:
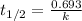
where,
 = half life period of first order reaction = ?
= half life period of first order reaction = ?
k = rate constant =
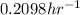
Putting values in above equation, we get:
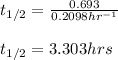
Hence, the half life of the sample of silver-112 is 3.303 hours.