Given :
10g of MgF₂ are dissolved in 2.2L of water.
To Find :
The molarity of this solution.
Solution :
Molar mass of MgF₂ is :
M = 24 + 19 + 19 g/mol
M = 62 g/mol
Moles in 10 g of MgF₂, n = 10/62 = 0.161 moles.
Now, we know molarity is given by :
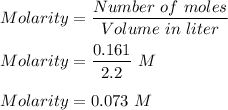
Therefore, the molarity of solution is 0.073 M.