Answer:

Step-by-step explanation:
First, we must find the density of the unknown metal.
Density is found by dividing the mass by the volume.
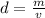
The mass of the metal is 89 grams and the density is 10 cubic centimeters.
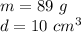
Substitute the values into the formula.
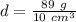
Divide.
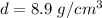
Now we know the density and can identify the unknown metal.
- Nickel: 8.9 g/cm³
- Silver: 10.5 g/cm³
- Lead: 11.35 g/cm³
- Mercury: 13.55 g/cm³
The density matches nickel's density. Therefore, this metal must be nickel.