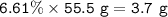Mass of Hydrogen = 3.7 g
Further explanation
The empirical formula is the smallest comparison of atoms of compound forming elements.
A molecular formula is a formula that shows the number of atomic elements that make up a compound.
(empirical formula) n = molecular formula
180.2 g/mol of glucose-C₆H₁₂O₆ contains 72.1 grams of carbon, 96 g/mol of oxygen and the remainder is hydrogen
Remainder Hydrogen :
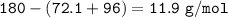
% mass of Hidrogen in Gllucose :
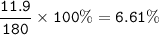
So mass of Hydrogen in 55.5 g Glucose :