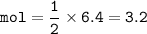Moles of Sulfuric acid needed to react completely : 3.2
Further explanation
A reaction coefficient is a number in the chemical formula of a substance involved in the reaction equation. The reaction coefficient is useful for equalizing reagents and products.
Reaction (balanced)
H₂SO₄ (aq) + 2 LiOH (aq) ⇒ Li₂SO₄ (aq) + 2 H₂O (l)
moles of LiOH = 6.4
From equation, mol ratio of H₂SO₄ : LiOH = 1 : 2, so mol H₂SO₄ :