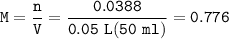The concentration (molarity) of [H₃O⁺]=0.776 M
Further explanation
We balance the reaction first so that the number of atoms and the charge of the product and reactant are the same
Reaction(balanced) :
H₂S⇒2H⁺+S²⁻ (eq 1)
2H⁺+6H₂O⇒4H₃O⁺+2OH⁻ (eq 2)
mass of H₂S=0.33 g
mol H₂S (MW=34 g/mol) :
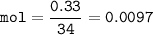
From equation 1, mol H⁺ = 2 x mol H₂S , so mol H⁺ =
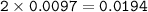
From equation 2, mol H₃O⁺ = 2 x mol H⁺(4 : 2) , so mol H₃O⁺ :
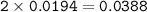
Or simply mol H₃O⁺ = 4 x mol H₂S = 4 x 0.0097 = 0.0388
the molarity of [H₃O⁺] =