Answer: 324 g (to 3 sf)
Step-by-step explanation:
Based on the coefficients of the equation, we know that when 3.00 mol of water is consumed, 3.00 mol of carbon dioxide is also consumed.
- The atomic mass of carbon is 12.011 g/mol.
- The atomic mass of oxygen is 15.9994 g/mol.
Thus, the formula mass of carbon dioxide is:
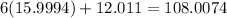 g/mol.
g/mol.
Thus, 3.00 mol has a mass of (108.0074)(3.00)=324 g (to 3 sf)