Given :
Number of molecules of
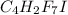 .
.
To Find :
How many moles are in given number of molecules.
Solution :
We know, in 1 moles of any element/compound contains
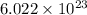 at atoms/molecules.
at atoms/molecules.
So, number of moles in
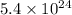 molecules are :
molecules are :
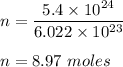
Therefore, number of moles are 8.97 .