Answer:
5.61 e⁻³
Step-by-step explanation:
Given that:
The barrier length = 0.681 nm = 6.81 × 10⁻¹⁰
The difference between the height of the barrier & the energy of the electron is;
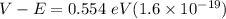
= 8.864 × 10⁻²⁰ V
where;
m = 9.1 × 10⁻³¹ kg
The probability
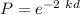
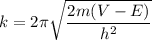
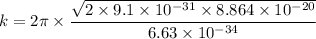
k = 3.806 × 10⁹
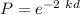
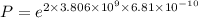
P = 0.005606
P = 5.61 × 10⁻³
P = 5.61 e⁻³