Answer:
The specific heat of the metal is 0.212 J/(g°C).
Step-by-step explanation:
We can calculate the specific heat of the metal by the following equilibrium:
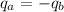

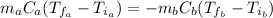
In the above equation, we have that the heat loses by the metal (b) is gained by the water (a).
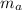 : is the water's mass = 72.0 g
: is the water's mass = 72.0 g
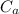 : is the specific heat of water = 4.184 J/(g°C)
: is the specific heat of water = 4.184 J/(g°C)
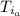 : is the initial temperature of the water = 19.2 °C
: is the initial temperature of the water = 19.2 °C
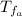 : is the final temperature of the water = 25.5 °C
: is the final temperature of the water = 25.5 °C
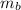 : is the metal's mass = 141 g
: is the metal's mass = 141 g
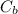 : is the specific heat of metal =?
: is the specific heat of metal =?
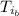 : is the initial temperature of the metal = 89.0 °C
: is the initial temperature of the metal = 89.0 °C
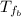 : is the final temperature of the water = 25.5 °C
: is the final temperature of the water = 25.5 °C
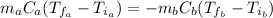
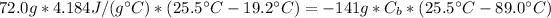
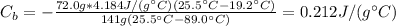
Therefore, the specific heat of the metal is 0.212 J/(g°C).
I hope it helps you!