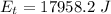Answer:
The value is
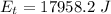
Step-by-step explanation:
From the question we are told that
The atmospheric temperature is
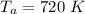
The molar mass of carbon dioxide is
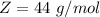
The pressure is
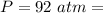
The number of moles is
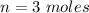
Generally the translational kinetic energy is mathematically represented as
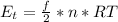
Here R is the gas constant with value
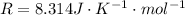
Generally the degree of freedom of carbon dioxide in terms of translational motion is f = 3
So
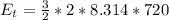
=>