Answer:
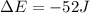
Step-by-step explanation:
Hello!
In this case, since the first law of thermodynamics states that the heat added to system and the work done by the system are able to define the change in the internal energy as follows:
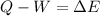
Since this problem shows that the system releases 113.0 J of heat, we use it negative as well as the 61.0 J of work performed on the system; therefore we write:
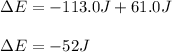
Best regards!