Answer:
B). the temperature of B increases more than the temperature of A
Step-by-step explanation:
For constant volume process, relation between molar heat capacity and temperature is given by,
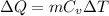
where, ΔQ is the amount of heat required
m is the mass of the gas
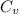 is heat capacity at constant volume
is heat capacity at constant volume
ΔT is the change in temperature.
For a constant energy ΔQ,
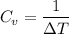
Given,
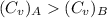
∴
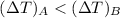
Thus the temperature of gas B increases more than the temperature of gas A.