Answer:
The average speed of molecule of oxygen is one-fourth of the speed of the hydrogen molecule.
Step-by-step explanation:
The kinetic energy of gas molecule is given by following equation:
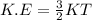
Since the temperature (T) is constant for both molecules. Therefore, there kinetic energies will also be the same. Therefore,
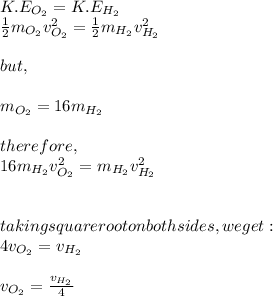
The average speed of molecule of oxygen is one-fourth of the speed of the hydrogen molecule.