Answer:
-4 is the coefficient.
Explanation:
We are given the expression:
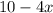
===================================
A coefficient is a term that is multiplied by a variable.
In the expression given, '-4' is getting multiplied by 'x'.
This means that '-4' would be the coefficient in the equation.
===================================
Hope this helps.