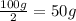Answer:
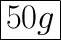
Definition:
Half-life- The time taken for half of the radioactive isotopes to decay.
Step-by-step explanation:
How does radioactive decay work? Radioactive decay is a process by which unstable nuclei become more stable through the emission of alpha or beta particles or gamma rays.
Since a half-life is the time taken for half of the isotopes to decay, we can simply divide the initial mass of 100 grams by 2; this gives us 50 grams.
1) Divide 100g by 2.