Answer:
1.12 moles of potassium
6.76x10²³ atoms of potassium
Step-by-step explanation:
In order to convert g of potassium to moles, we need to use its molar mass (which we can find via the periodic table):
- Molar Mass of K = 39.09 g/mol
- 43.9 g K ÷ 39.09 g/mol = 1.12 mol K
Then, to convert moles into atoms, we use Avogadro's number (6.023x10²³ atoms per mole):
- 1.12 mol K * 6.023x10²³
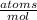 = 6.76x10²³ K atoms
= 6.76x10²³ K atoms