setup 1 : to the right
setup 2 : equilibrium
setup 3 : to the left
Further explanation
The reaction quotient (Q) : determine a reaction has reached equilibrium
For reaction :
aA+bB⇔cC+dD
![\tt Q=(C]^c[D]^d)/([A]^a[B]^b)](https://img.qammunity.org/2021/formulas/chemistry/high-school/9tblzc1nv4msqnblpr8dd8g9ixl97f45uz.png)
Comparing Q with K( the equilibrium constant) :
K is the product of ions in an equilibrium saturated state
Q is the product of the ion ions from the reacting substance
Q <K = solution has not occurred precipitation, the ratio of the products to reactants is less than the ratio at equilibrium. The reaction moved to the right (products)
Q = Ksp = saturated solution, exactly the precipitate will occur, the system at equilibrium
Q> K = sediment solution, the ratio of the products to reactants is greater than the ratio at equilibrium. The reaction moved to the left (reactants)
Keq = 6.16 x 10⁻³
Q for reaction N₂O₄(0) ⇒ 2NO₂(g)
![\tt Q=([NO_2]^2)/([N_2O_4])](https://img.qammunity.org/2021/formulas/chemistry/high-school/fpidzfest7qmiq5tgjs7f3n18g6qfdmejk.png)
Setup 1 :
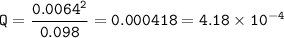
Q<K⇒The reaction moved to the right (products)
Setup 2 :
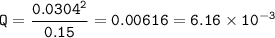
Q=K⇒the system at equilibrium
Setup 3 :
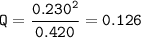
Q>K⇒The reaction moved to the left (reactants)