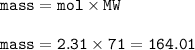Mass of Cl₂ : 164.01 g
Further explanation
A mole is a number of particles(atoms, molecules, ions) in a substance
This refers to the atomic total of the 12 gr C-12 which is equal to 6.02.10²³, so 1 mole = 6.02.10²³ particles
Can be formulated :
N = n x No
N = number of particles
n = mol
No = 6.02.10²³ = Avogadro's number
mol Cl₂ :

mass Cl₂(MW=71 g/mol) :